Spring Soil Nitrogen Following The Drought Of 2012
URBANA, ILL.
Last fall, with funding provided
through the Illinois
Council for Best Management
Practices (C-BMP),
GROWMARK, C-BMP, and the
University of Illinois initiated
the N-Watch soil sampling
program to see how much inorganic
N remained in the soil
following the drought of 2012.
Fall sampling revealed fairly high amounts of
soil N, with 151 samples statewide averaging
19.5 ppm of nitrate-N in the top foot of soil. We
multiply this time 4 to get lb of N per acre, so
soils represented by these samples had an average
of 78 lb of nitrate-N per acre. Samples
from northern Illinois had higher levels (26
ppm) than those from central and southern Illinois
(both 18 ppm), even though 2012 corn
yields in northern Illinois were higher than in
central or southern Illinois. The second foot of
soil depth had more than 15 ppm of nitrate-N,
which meant another 62 lb of nitrate-N per acre,
for a total of 140 lb N per acre.
One reason for fall sampling is to inventory
soil N in order to know the potential for loss of
leftover soil N. If the weather stays dry through
the fall and winter, we expect minimal loss of
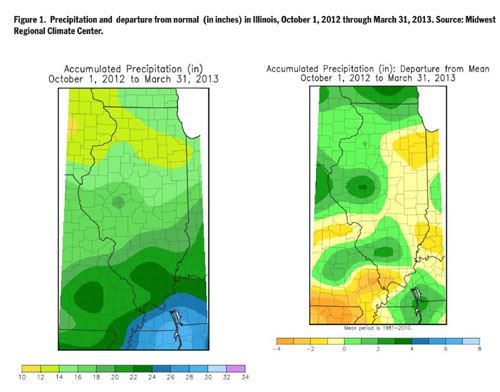
soil N. But precipitation returned to normal over
most of Illinois during the past 6 months, most
areas showing only small departures from normal
over the October 2012 to March 2013 period
(Figure 1). Normal precipitation from
October 1 through March ranges from about 12
inches in the northern edge of Illinois to about
20 inches at the southern tip of the State.
While nitrate-N moves readily down into the
soil profile as water moves down through the
soil, the lack of rainfall during the 2012 growing
season meant that nitrate from fertilizer N and
from soil organic matter simply accumulated.
Water only moves as far down as it takes to wet
the soil, and in a very dry soil, it can take 6 to
10 inches or more of water to wet the soil. Even
more water is needed to move through to deeper
layers and into tile lines. Indications are that
most tile lines in Illinois have been running for
the past few weeks in the areas that were dry
longer, and for the past couple of months in
areas that received more rain and rain starting
earlier.
Not surprisingly, a few reports in recent weeks
from sampling tile line outflow show elevated
levels of nitrate-N. This is normal for spring outflow,
but with little or no tile line outflow as N
accumulated last summer and fall, and with the
large amounts of nitrate we found in fall sampling,
the flush of nitrate-N may be larger than
normal this spring.
How much soil N has been lost?
Dan Schaefer of C-BMP took both fall 2012
and spring (March) 2013 samples in a few fields
in east central Illinois that can give us some estimates
of N loss. In one field where N was applied
in the early spring in 2012, October
samples had about 16 and 13 ppm of nitrate-
N in the top and second foot of depth. In mid-
March 2013, those numbers had fallen to 6 and
10 ppm, respectively. Assuming no net conversion
of N from ammonium to nitrate, this reflects
a loss of about 50 lb of nitrate from the
top two feet. Much of this probably remains in
the soil below the top 2 feet, but some may have
entered the drainage tile by now.
In a second field in which N was applied as
spring sidedressed ammonia, nitrate-N went
from 24 and 8 ppm in the first and second foot
of soil last October, to 9 and 18 ppm, respectively,
by mid-March of this year. That represents
a net loss of only about 20 lb of N per
acre, but a considerable amount of movement
of nitrate from the top to the second foot. Nitrate
losses have possibly been limited because it
took so long (and so much precipitation) for dry
soils to rewet to the point that water started to
move through the profile.
Ammonium-N is immobile in soil, but in warm
soils, microbes generally convert it rather
quickly to nitrate-N. Because of this we normally
see only 2 or 3 ppm of ammonium-N in
either the fall or the spring. We found an average
of about 5 ppm of ammonium-N in both the
top and second foot of soil in the 2012 fall samples.
In the second field described above, the fall
sample had about 11 ppm of ammonium-N in
the top foot. This could have come from spring-applied
ammonia or from mineralization of soil
organic matter after rain in September. The surprise
is that the March sample still had 9 ppm
of ammonium-N in the top foot. It is clear that
there was limited net conversion of ammonium
to nitrate in the top foot of soil between mid-October
and mid-March.
Does this mean that ammonia applied in the
fall of 2012 is still present? Most of the ammonia
was applied after soil temperatures had
dropped to below 50. Soils also stayed relatively
cool, with average soil temperatures at the 4-
inch depth of 49, 45, 34, and 34 degrees in November,
December, January, and February,
respectively. In contrast, 4-inch average soil
temperatures in January and February were 39
and 41.5 degrees, respectively, in 2012. There
were only 3 days in January and February 2013
with average 4-inch soil temperature of 40 degrees
or more. So we think that soil temperatures
have stayed low enough this winter to
minimize the conversion of ammonium to nitrate.
We don’t have many direct measures of how
much ammonia applied in the fall of 2012 is still
present, but based on a few estimates, we believe
that loss of NH3 applied in fall 2012 has
been relatively small. One field that Dan Schaefer
sampled in October 2012 had 11.5 and 7.6
ppm of nitrate-N, and 3.7 and 2 ppm of ammonium-
N in the top and second foot, respectively.
In November, 100 lb of N as NH3 was applied.
March samples showed 6.5 and 11 ppm nitrate-
N and 12.7 and 2.4 ppm of ammonium-N in the
top and second foot, respectively. Probe samples
from the application band are not a very reliable
way to measure N, but if we assume a 35-lb loss
of nitrate-N (average of the other two fields described
above), the probe sample “found” about
2/3rds of the N that was applied last fall, and
about half of that remains in the ammonium
form. Compared to some fall-to-spring changes
in ammonium-N reported in the literature, we
think that nitrification (conversion of ammonium
to nitrate) and loss of fall-applied N have
been less than normal this winter.
Other sampling done over the past month, in
some cases to see if winter wheat should have N
topdressing rate adjusted, has shown nitrate-
N to be fairly low, often less than 5 or 6 ppm.
The winter wheat crop would not have taken up
much N when samples were taken, but soil nitrate-
N does drop by 1 ppm for each 4 lb. of N
present in the wheat (or other) crop at the time
of sampling.
Spring Sampling, 2013
The priority for spring sampling should be
those fields where corn in 2013 will follow corn
(from which fall samples were taken) in 2012.
We suggested last fall that any spring sampling
done in order to adjust rates for the 2013 corn
crop should best be done close to corn planting
time, or at side-dress time. However, it would be
useful if at least some of those sites where fall
soil N levels were high could be sampled within
the next few weeks so that we could better
guess how much additional sampling would be
useful.
I suggest that sites with fall nitrate-N above 25
ppm nitrate-N in the top foot (that is about 25
percent of Illinois sites) be sampled first, but
any and all sites can be re-sampled if volunteers
are willing to do this. All those who coordinated
sampling last fall and who requested forms and
shipping materials through me will get an email
in the next few days with brief instructions. The
process will be the same as it was last fall, with
samples sent to A&L for analysis, and funding
provided through C-BMP. Those who sampled
under GROWMARK’s direction last fall will continue
in that program this spring.
Those who did not sample fields last fall but
would like to do so this spring are also invited to
participate. Instead of sending requests through
me, however, we ask that those sampling this
spring for the first time send requests to Jean
Payne jeanp@ifca.com at the Illinois Fertilizer &
Chemical Association, who will pass along the
request for sampling materials and instructions.
It might be difficult to get the deep (1 to 2-ft)
samples in fields where soils remain wet. We
think the 1-ft sample will show us how much N
rate adjustment might be appropriate. Those
willing and able to take samples from the second
foot are encouraged to do so, however.
It is best to take samples before any N has
been applied in the spring, though we can avoid
the band if, for example, some starter was used
at planting and sampling is done after that.
Combine in a bucket enough samples to represent
the area you want to represent, and take a
subsample of this to send to the lab for analysis.
Soils should be sent for analysis as soon as possible,
and kept refrigerated if needed, to minimize
N transformations before analysis.
Adjusting N rates based on spring sampling
The pre-sidedress N test (PSNT) and the preplant
N test (PPNT) were developed to make N
rate adjustments based on N already present in
the spring. Adjustments are not generally suggested
if the soil has less than 10 ppm of nitrate-
N in the top 6-7 inches (10 ppm is about
20 lb. of nitrate-N per acre), and no additional
fertilizer N is suggested if the surface soil has
more than 25 ppm nitrate-N (some states use
20 and some use 30 ppm as this limit). Fertilizer
N rates are decreased as surface soil nitrate-N
increases from 10 to 25 ppm.
Because of uncertainty in sampling, many
universities suggest making adjustments as
ranges; for example, the N application rate
might be reduced by 30 to 50 lb. N per acre if
the soil has 10 to 15 ppm of nitrate-N; by 60 to
120 lb. N if the soil has 15 to 20 ppm, etc. In
practice, adjusting N rate based on nitrate-N
present has often been most useful in fields
where a lot of organic N – from manure or forage
legumes were grown previously – was added, in
which case it is a test for how much N mineralized.
Spring soil N testing also requires sampling
and then waiting for results during a busy
time. But it can be used if we suspect that there
are more than normal amounts of soil N. In
cases where there has been wet weather and
some N loss, it can also be used to help decide
whether or not to make a supplemental N application.
While the University of Illinois has not actively
promoted the use of the PPNT or PSNT, it is logical
to apply less than full N rates if spring samples
show an appreciable amount of soil N
already present. We think that a reasonable way
to do this is to calculate lb. nitrate-N per acre
(ppm of nitrate-N in the top foot times 4) and to
subtract this from the normal N rate. It may be
safer not to make any adjustments if nitrate-N
is less than 10 ppm, since we would consider
low levels to be normal. But as an example,
finding 20 ppm in a sample would suggest a reduction
of 80 lb. N per acre in the fertilizer N
rate. That’s conservative – 30 ppm would rule
out any more N under the PSNT guidelines in
use, but would mean lowering rate by 120 lb.
based on what we’re proposing here.
Sampling uncertainty does mean some uncertainty
in N rate adjustments. That’s a concern,
especially if we sample only a small area in the
field. So spring resampling in only the small
area where fall samples were taken under the
N-Watch program, while it meets the important
objective of measuring changes from fall to
spring, may not be adequate for making adjustments
for a whole field. At minimum, samples
taken for N rate adjustments should be taken
from different soil types in a field to see if soil N
levels have enough consistency to warrant adjustments.
Δ
DR. EMERSON NAFZIGER: Professor/Research
Education Center Coordinator, University of Illinois